“`html
How to Effectively Find the Limiting Reactant in Chemical Reactions
Understanding how to identify the limiting reactant in chemical reactions is fundamental in the field of stoichiometry. The limiting reactant defines the maximum amount of product that can be formed, making its identification crucial for maximizing reaction yield. This article will guide you through the steps of finding the limiting reactant in any chemical reaction, including the necessary calculations and methodologies.
Understanding the Basics of Limiting Reactants
Before diving into the reactant calculation, it’s important to grasp what a limiting reactant is. In any chemical reaction, reactants combine in fixed proportions according to their balanced equation. The reactant that is completely consumed first determines the extent of the reaction – hence, it is known as the limiting reactant. The other reactants present are described as excess reactants because they remain unreacted once the limiting reactant is gone.
Identifying Reactants and Their Roles
In every chemical equation, the reactants interact to form products. To identify the limiting reactant, you must know the initial amounts of each reactant involved in the reaction. For instance, in the reaction: A + B → C, if you start with more of A than needed to react with all of B, then B is the limiting reactant. Understanding reactant proportions can help streamline the reaction comparisons.
Using the Balanced Equation
A critical step in determining the limiting reactant is deriving a balanced equation for the reaction. Each reactant’s coefficients in the balanced equation establish the mole ratio necessary for calculations. For example, if the equation is \(2A + B → C\), two moles of A react with one mole of B. This stoichiometric relationship helps in assessing how much of each reactant is needed and consumed during the reaction.
Calculation Methods for Identifying the Limiting Reactant
After establishing the balanced equation, the next step involves calculating the amount of product that can result from each reactant using their respective initial reactant amounts. This is where you will execute stoichiometric calculations to predict the maximal product formation.
Step-by-Step Calculations
To determine the limiting reactant, apply the following process:
1. Write the balanced equation.
2. Convert all given reactant amounts into moles. Use the molar mass determination for conversion.
3. For each reactant, use the balanced equation to calculate how many moles of product can be formed.
4. The reactant that produces the least amount of product is your limiting reactant.
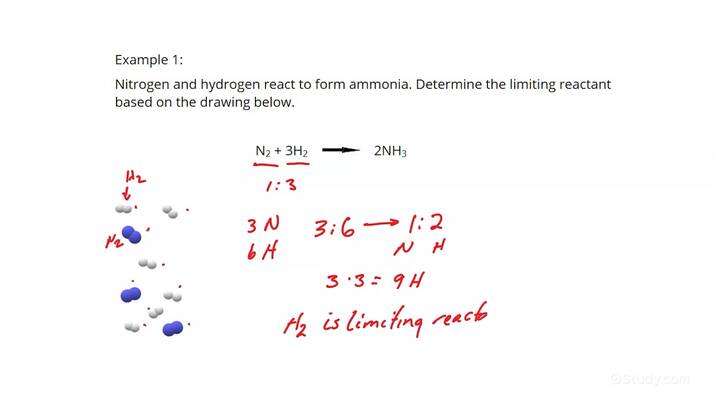
By following these steps, you can accurately predict how much of the product will be formed, allowing for efficient use of resources in any laboratory experiment.
Example Calculation
Let’s consider an example: If you react 4 moles of A with 3 moles of B according to the equation \(2A + B → 2C\), first, determine how many moles of C could be formed:
– From A: 4 moles of A can produce 4 moles of C (since 2 moles of A yield 2 moles of C).
– From B: 3 moles of B can produce 6 moles of C (since 1 mole of B yields 2 moles of C).
Since only 4 moles of C can be formed from limited A, A is the limiting reactant.
Excess Reactant and Its Implications
Understanding the role of excess reactants is equally important. While these reactants remain after the reaction completes, their presence does not impact the maximum product yield but is crucial for determining theoretical vs actual yield during experiments.
Calculating Excess Reactant Amounts
After identifying the limiting reactant, you might want to calculate how much of the excess reactant remains unreacted. Use the same balanced equation and the limiting reactant amount to find the excess remaining. For instance, with 4 moles of reactant A and 3 moles of reactant B, if A is limiting, then calculate the leftover B based on how many moles reacted with A fully.
Conclusion and Key Takeaways
Identifying the limiting reactant is essential for optimizing reactions and maximizing yields. Understanding reactant constraints, mole ratios, and performing quantitative analysis helps in efficiently managing resources during experiments. By mastering these methods, you will enhance your skills in chemical synthesis and advance your knowledge of chemistry principles.
Key Takeaways
- The limiting reactant restricts product formation.
- Balanced equations are crucial for accurate calculations.
- Using stoichiometric coefficients assists in determining reactant amounts.
- Calculate excess reactants for comprehensive yield analysis.
FAQ
1. What methods can be used to determine the limiting reactant?
Two primary methods are commonly used: firstly, the stoichiometric calculation method, which involves converting reactant amounts into moles and applying the mole ratio from the balanced equation; secondly, a comparison of the potential product yield from each reactant helps clearly indicate the limiting reagent.
2. How do you calculate excess reactants?
You can calculate excess reactants by subtracting the amount that reacts (based on the limiting reactant) from the initial amount. This allows you to know how much of the excess reactant remains unreacted after the reaction.
3. Why is understanding limiting vs excess reactants important in chemical experiments?
Grasping the dynamics of limiting and excess reactants is vital as it enables accurate predictions of yields and ensures efficient use of materials. It helps in improving the quality and effectiveness of laboratory experiments.
4. What are common laboratory techniques to apply these concepts?
Common techniques include titration methods for reactant concentration analysis, quantitative transfers for accurate measuring, and spectroscopic methods to validate reactions and product formation, applying analytical chemistry principles.
5. Can the limiting reactant change in repeated experiments?
Yes, the limiting reactant can differ based on the starting amounts of each reactant. Changes in initial concentrations can lead to different outcomes and thus might alter which reactant is limiting in the reaction.
“`
